In the UK, Translation services for Medical Device Manuals UK are paramount for patient safety and regulatory compliance. Professional translators with medical expertise ensure accurate communication of complex device instructions, fostering trust among healthcare professionals and users. High-quality translations meet MHRA standards, prevent misuse, and promote legal adherence, making these services essential for medical device manufacturers aiming to serve diverse linguistic and cultural markets.
In the medical device industry, clear and precise user manuals are paramount. Ensuring that instructions are easily understandable across diverse user groups not only enhances safety but also fosters trust in the product. This article delves into the significance of unambiguous English manuals, highlighting challenges in conveying complex information accurately. We explore the pivotal role of professional translation services in the UK, focusing on quality and consistency guarantees. Additionally, best practices for crafting effective user manuals are provided to optimize usability. Translation services for medical device manuals in the UK play a crucial part in achieving these goals.
- Understanding the Importance of Clear Manuals in Medical Device Industry
- Challenges in Communicating Complex Information Accurately
- The Role of Professional Translation Services in UK
- Ensuring Quality and Consistency in Technical Document Translation
- Best Practices for Creating Effective User Manuals for Medical Devices
Understanding the Importance of Clear Manuals in Medical Device Industry
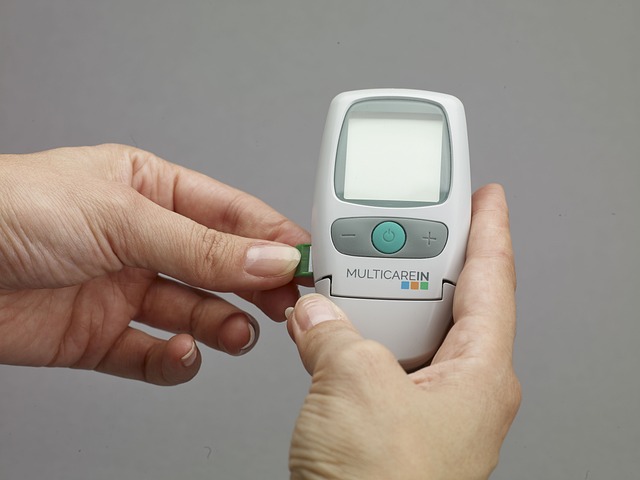
In the medical device industry, clear and precise documentation is not just a best practice—it’s a matter of life and death. Medical devices often come with complex instructions for use, maintenance, and safety protocols. When these manuals are poorly translated or lack clarity, it can lead to serious consequences, including incorrect device usage, malfunctions, and even patient harm. That’s why ensuring your device manuals are written in fluent, error-free English is paramount, especially when catering to a global market like the UK.
Translation services for Medical Device Manuals UK play a crucial role here. Professional translators with medical expertise can bridge the gap between technical jargon and everyday language, making critical information accessible to healthcare professionals and patients alike. Accurate translations not only comply with regulatory requirements but also enhance user safety and device adoption, ultimately fostering trust in your brand.
Challenges in Communicating Complex Information Accurately

Communicating complex information accurately is one of the primary challenges in crafting device manuals, especially when targeting a global audience. Medical device manuals often deal with intricate technical details, safety protocols, and usage guidelines that demand precision in translation and explanation. The challenge lies in ensuring that these instructions are not only accurately translated but also easily comprehensible across different linguistic and cultural backgrounds.
In the UK, where medical devices are subject to stringent regulations, clear communication is paramount. Translation services for medical device manuals play a crucial role in bridging this gap. Professional translators with expertise in medical terminology must be engaged to avoid critical mistakes. Mistranslations or vague explanations can lead to misuse of devices, safety hazards, and even legal consequences. Therefore, investing in high-quality translation services is not just an option but a necessity for manufacturers aiming to provide clear, precise, and reliable manuals in English.
The Role of Professional Translation Services in UK

In the UK, where a diverse range of languages is spoken, ensuring clear communication in English is paramount, especially when it comes to device manuals for medical equipment. This is where professional translation services play a vital role. Medical device manufacturers often rely on these experts to translate their user manuals and documentation into accurate and accessible language for their multilingual audience.
Translation services for medical device manuals UK-based companies offer ensure that instructions are not only linguistically correct but also culturally adapted, adhering to local regulations and terminology standards. This is crucial in a country like the UK, where medical devices must comply with stringent guidelines, such as those set by the Medicines and Healthcare products Regulatory Agency (MHRA). Professional translators possess the specialized knowledge to convey complex medical information accurately, ensuring user safety and compliance with legal requirements.
Ensuring Quality and Consistency in Technical Document Translation
When it comes to technical document translation, especially for medical device manuals in the UK, ensuring quality and consistency is paramount. Accurate translation services should not only convey the exact meaning of the original content but also adhere to industry-specific terminology to maintain precision. Medical devices have specific jargon and regulatory requirements, making it crucial to work with professional translators who understand these nuances.
Translation service providers must employ rigorous quality control measures to guarantee consistency across all translated documents. This includes thorough proofreading, editing, and review processes to catch any errors or inconsistencies. Using translation memory tools can also help maintain terminological consistency, ensuring that the same terms are used throughout various parts of the manual, thereby providing a seamless reading experience for users.
Best Practices for Creating Effective User Manuals for Medical Devices
Creating effective user manuals for medical devices requires a meticulous approach, especially in English, to ensure clear communication and accurate understanding among diverse users. Here are some best practices to achieve this:
When preparing device manuals, accuracy is paramount. Medical terminology can be intricate, so employing professional translators or editors with expertise in both the subject matter and the target language (in this case, English) is vital. Translation services for medical device manuals in the UK should be your first step to guarantee precision. These specialists will ensure that complex instructions and descriptions are conveyed accurately, without losing their clarity. Additionally, maintaining a consistent tone throughout the manual is essential for better user engagement.
In ensuring patient safety and regulatory compliance, clear and precise English manuals are paramount in the medical device industry. Overcoming challenges in conveying complex information accurately is crucial. Professional translation services in the UK play a vital role in this process, maintaining quality and consistency across global markets. Adhering to best practices for creating effective user manuals further emphasizes the importance of accessible and understandable documentation. When seeking translation services for Medical Device Manuals UK, professionals with expertise in medical terminology and regulatory requirements are essential to deliver accurate and life-saving translations.
